New strategy for synthesis of hydroxyl-terminated poly(3-hexylthiophene) and its block copolymer using an η3-π-allyl hydroxyl-functionalized Ni(i) initiator†
Abstract
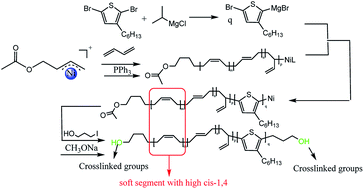
A novel hydroxyl-terminated π-allyl nickel initiator [η3-Ni(CH2CHCHCH2OCOCH3)][BPhF4] is synthesized for the first time. 1H-NMR analysis confirms that the Ni initiator possesses a typical π-allyl structure with a protected hydroxyl group. The Ni initiator is applied to synthesize hydroxyl-terminated poly(3-hexylthiophene). It successfully initiates the homopolymerization of thiophene, and yields narrow molecular weight distribution, hydroxyl-terminated poly(3-hexylthiophene). Moreover, it is found that [η3-Ni(CH2CHCHCH2OCOCH3)][BPhF4] has high initiating activity in the polymerization of styrene and butadiene. A hydroxyl-terminated polybutadiene-b-poly(3-hexylthiophene) block copolymer is synthesized by using [η3-Ni(CH2CHCHCH2OCOCH3)][BPhF4] to improve the workability and brittleness of poly(3-hexylthiophene).


 Please wait while we load your content...
Please wait while we load your content...