Study on the performance of NiO/ZnxZr1−x catalysts for CO2 hydrogenation
Abstract
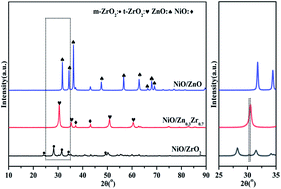
The NiO/ZnxZr1−x (x represents the molar mass of Zn) catalyst was prepared by the impregnation method and tested in CO2 methanation. The activity results show that NiO/Zn0.3Zr0.7 has a higher CO2 conversion rate and methane selectivity than NiO/ZnO and NiO/ZnO–ZrO2. Combined with N2 adsorption–desorption, H2-TPR, CO2-TPD, H2-TPD, XRD, TEM, XPS and FTIR and other characterization methods, the physical and chemical properties of NiO/ZnO–ZrO2 were studied. The incorporation of ZnO into NiO/ZrO2 forms a ZnO–ZrO2 solid solution, and the combination of the solid solution weakens the interaction between NiO and the oxide support, thereby promoting the reduction and dispersion of NiO. The H2-TPR experiment results show that, because ZnO–ZrO2 forms a solid solution, NiO is better dispersed on the surface, resulting in a significant reduction in the reduction temperature of NiO. Using FTIR to conduct CO2 adsorption and methanation experiments on NiO/ZnxZr1−x to determine the adsorbed species and intermediates, the results show that CO2 methanation follows the formate pathway.


 Please wait while we load your content...
Please wait while we load your content...