The photoelectron-imaging spectroscopic study and chemical bonding analysis of VO2−, NbO2− and TaO2−†
Abstract
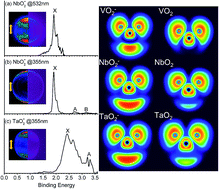
The transition-metal di-oxides, namely VO2−, NbO2− and TaO2− have been studied using photoelectron velocity map imaging (PE-VMI) in combination with theoretical calculations. The adiabatic electron affinities of VO2−, NbO2− and TaO2− are confirmed to be 2.029(8), 1.901(10) and 2.415(8) eV, respectively. By combining Franck–Condon (FC) simulation with theoretical calculations, the vibrational feature related to Nb–O and Ta–O stretching modes for the ground state has been unveiled. The photoelectron angular distribution (PAD) for VO2−, NbO2− and TaO2− is correlated to the photo-detachment of the highest occupied molecular orbitals (HOMOs), which primarily gets involved in s- and d-orbitals of the V, Nb and Ta atoms. A variety of theoretical calculations have been used to analyze the chemical bonding features of VO2−1/0, NbO2−1/0 and TaO2−1/0, which show that the strong M–O (M = V, Nb and Ta) bond is mainly characterized as ionicity.


 Please wait while we load your content...
Please wait while we load your content...