Melamine-based functionalized graphene oxide and zirconium phosphate for high performance removal of mercury and lead ions from water†
Abstract
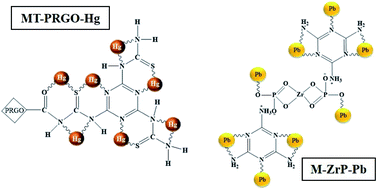
Heavy metal ions are highly toxic and widely spread as environmental pollutants. This work reports the development of two novel chelating adsorbents, based on the chemical modifications of graphene oxide and zirconium phosphate by functionalization with melamine-based chelating ligands for the effective and selective extraction of Hg(II) and Pb(II) from contaminated water sources. The first adsorbent melamine, thiourea-partially reduced graphene oxide (MT-PRGO) combines the heavier donor atom sulfur with the amine and triazine nitrogen's functional groups attached to the partially reduced GO nanosheets to effectively capture Hg(II) ions from water. The MT-PRGO adsorbent shows high efficiency for the extraction of Hg(II) with a capacity of 651 mg g−1 and very fast kinetics resulting in a 100% removal of Hg(II) from 500 ppb and 50 ppm concentrations in 15 second and 30 min, respectively. The second adsorbent, melamine zirconium phosphate (M-ZrP), is designed to combine the amine and triazine nitrogen's functional groups of melamine with the hydroxyl active sites of zirconium phosphate to effectively capture Pb(II) ions from water. The M-ZrP adsorbent shows exceptionally high adsorption affinity for Pb(II) with a capacity of 681 mg g−1 and 1000 mg g−1 using an adsorbent dose of 1 g L−1 and 2 g L−1, respectively. The high adsorption capacity is also coupled with fast kinetics where the equilibrium time required for the 100% removal of Pb(II) from 1 ppm, 100 ppm and 1000 ppm concentrations is 40 seconds, 5 min and 30 min, respectively using an adsorbent dose of 1 g L−1. In a mixture of six heavy metal ions at a concentration of 10 ppm, the removal efficiency is 100% for Pb(II), 99% for Hg(II), Cd(II) and Zn(II), 94% for Cu(II), and 90% for Ni(II) while at a higher concentration of 250 ppm the removal efficiency for Pb(II) is 95% compared to 23% for Hg(II) and less than 10% for the other ions. Because of the fast adsorption kinetics, high removal capacity, excellent regeneration, stability and reusability, the MT-PRGO and M-ZrP are proposed as top performing remediation adsorbents for the solid phase extraction of Hg(II) and Pb(II), respectively from contaminated water.


 Please wait while we load your content...
Please wait while we load your content...