Role of the backbone of nucleic acids in the stability of Hg2+-mediated canonical base pairs and thymine–thymine mispair: a DFT study†
Abstract
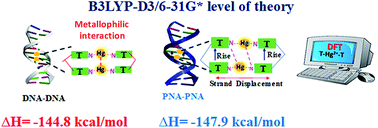
Metal-mediated base pairs have attracted attention in nucleic acid research and molecular devices. Herein, we report a systematic computational study on Hg2+-mediated base pairs with canonical and TT mispair dimers. The computed results revealed that the model DTTD (thymine–thymine with DNA backbone) mispair is more energetically favored than the canonical base pairs. The DTTTTD mispair dimer is more energetically stable by ∼36.0 kcal mol−1 than the corresponding canonical DATGCD base pairs. The Hg⋯Hg metallophilic interaction was observed with the DTTTTD mispair and not the canonical base pairs. The DATGCD (adenine: thymine, guanine: cytosine) base pairs were significantly perturbed upon interaction with the mercury ion; however, the TTTT mispairs were aligned upon interaction with the Hg2+ ion. The DTTTTD mispair adopts a B-type conformation with proper alignment of its nucleobases along the axis. The MESP calculations showed a larger Vmin value for the interacting nitrogen centers of the thymine nucleobase, supporting its stronger binding with the Hg2+ ion compared to the other nucleobases. The role of the backbone is crucial in nucleic acids to determine many useful properties, and PNAs have been exploited extensively in the literature. Thus, this study was further extended to metal-mediated PNA-containing dimer mispairs such as DTTTTP (thymine–thymine dimer model with hybrid DNA and PNA backbone) and PTTTTP (thymine–thymine dimer model with PNA backbone). The calculated results showed that the PTTTTP PNA mispair is thermodynamically more stable than the canonical dimers. The enthalpy calculated for DTTTTD and PTTTTP at the B3LYP-D3/6-31G* level of theory showed that PTTTTP is ∼3.0 kcal mol−1 more stable than DTTTTD. The metallophilic interaction of Hg2+ ions in the PTTTTP mispair was not observed; however, the metal ions interact with the nitrogen of the thymine bases, presumably enhancing the stability of this mispair by strong electrostatic interactions. These interactions arise due to the P-type conformations of PNAs, which lack metallophilic interactions between the metal ions and can adopt a wider and more unwounded helix. The interaction of the mispair dimers with the explicit water molecules also showed a similar stability trend to that observed with the implicit solvation model. The metallophilic interaction (Hg⋯Hg) was found to be conserved in DTTTTD. The AIM analysis performed for these dimers revealed that the interactions are primarily electrostatic in nature. The UV-vis absorption spectra of the mispair systems calculated at the B3LYP-D3/6-31G* level of theory using the TD-DFT method in the aqueous phase suggested that the absorption maximum is located at a longer wavelength in the case of PTTTTP compared to the corresponding DTTTTD and can be a signature to identify the formation of metal-mediated nucleic acid systems.


 Please wait while we load your content...
Please wait while we load your content...