An improved 4′-aminomethyltrioxsalen-based nucleic acid crosslinker for biotinylation of double-stranded DNA or RNA†
Abstract
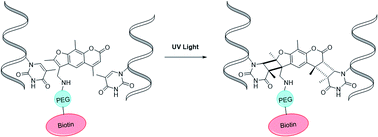
Nucleic acid crosslinkers that covalently join complementary strands of DNA/RNA have applications in both pharmaceuticals and as biochemical probes. Psoralen is a popular crosslinking moiety that reacts with double stranded DNA and RNA upon exposure to longwave UV light. The commercially available compound EZ-link psoralen-PEG3-biotin has been used in numerous studies to crosslink DNA and double-stranded RNA for genome-wide investigations. Here we present a new probe, AP3B, which uses the psoralen derivative, 4′-aminomethyltrioxsalen, to crosslink and biotinylate nucleic acids. We show that AP3B is 4 to 5 times more effective at labeling DNA in cells and produces a comparable number of crosslinks with over 100 times less compound and less exposure to UV light in vitro than EZ-link psoralen-PEG3-biotin.


 Please wait while we load your content...
Please wait while we load your content...