Porphyrin-based systems containing polyaromatic fragments: decoupling the synergistic effects in aromatic-porphyrin-fullerene systems†
Abstract
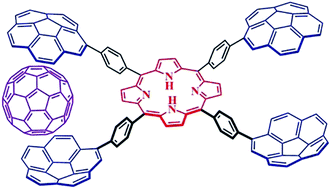
In this work, we report a two-step synthesis that allows the introduction of four pyrene or corannulene fragments at the para position of meso-tetraarylporphyrins using a microwave-assisted quadruple Suzuki–Miyaura reaction. Placing the PAHs at this position, further from the porphyrin core, avoids the participation of the porphyrin core in binding with fullerenes. The fullerene hosting ability of the four new molecular receptors was investigated by NMR titrations and DFT studies. Despite having two potential binding sites, the pyrene derivatives did not associate with C60 or C70. In contrast, the tetracorannulene derivatives bound C60 and C70, although with modest binding constants. In these novel para-substituted systems, the porphyrin core acts as a simple linker that does not participate in the binding process, which allows the system to be considered as two independent molecular tweezers; i.e., the first binding event is not transmitted to the second binding site. This behavior can be considered a direct consequence of the decoupling of the porphyrin core from the binding event.


 Please wait while we load your content...
Please wait while we load your content...