NMR-based quantitative studies of the conformational equilibrium between their square and folded forms of ascidiacyclamide and its analogues†
Abstract
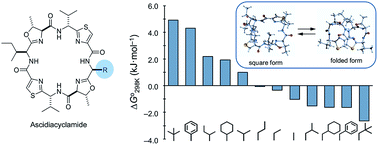
Ascidiacyclamide [cyclo(-Ile1,5-oxazoline2,6-D-Val3,7-thiazole4,8-)] (1) is a cytotoxic cyclic peptide from the ascidian, or sea squirt. Through structural analyses using asymmetric analogues [Xxx1: Ala (2), Val (3), Leu (4), Phe (5), cyclohexylalanine (6) and phenylglycine (7)], we previously showed 1 to exist in a conformational equilibrium between square and folded forms. In the present study, five new asymmetric analogues [Xxx1: 2-aminobutyric acid (8), 2-aminopentyric acid (9), tert-butylalanine (10), cyclohexylglycine (11) and tert-leucine (12)] were synthesized, and their structures were analyzed with X-ray diffraction and CD spectral measurements. Variable temperature 1H NMR measurements were performed to determine their equilibrium constants and their thermodynamic parameters. The use of two reference peptides made these quantitative studies possible. T3ASC, which contains three thiazole rings as a result of replacing oxazoline2 with thiazole, and dASC, in which the two oxazoline rings were deleted, were respectively used as square and folded reference peptides. The estimated parameters enabled more detailed discussion of the relationship between the bulkiness of substituents and the conformational free energies (ΔG°) of the peptides as well as the relationship between structure and cytotoxicity. The ΔG° values for peptides 1, 2, 3, 8, 9 and 11 decreased with decreases in the bulkiness of their substituents. We suggest that spontaneous folding is promoted as the bulkiness of substituents decreases. Peptides 7 and 12, which have large positive ΔG° values independently of temperature, did not exhibit spontaneous folding at any temperature; that is, their conformations were very stable in the square form. Peptides 4, 5, 6 and 10 had negative ΔG° values, despite their bulky substituents. Peptides with a positive ΔG° value showed cytotoxicity, and peptides with a negative ΔG° value showed reduced or no cytotoxicity. However, peptides 5 and 6 showed cytotoxicity equal to or stronger than 1. Those ten peptides except for 5 and 6 showed a clear structure–cytotoxicity relationship based on ΔG° values.


 Please wait while we load your content...
Please wait while we load your content...