Syntheses of tetrahydroquinoline-based chiral carbene precursors and the related chiral NHC–Au(i) complex†
Abstract
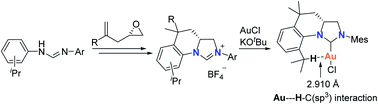
Four tetrahydroquinoline-based chiral carbene precursors were synthesized using unsymmetrical N,N′-diarylformamidines and chiral 2-allyloxiranes as starting materials. A representative NHC–gold complex has been prepared and fully characterized, the crystal structure of which reveals an intramolecular Au⋯H–C(sp3) interaction between Au(I) and the hydrogen atom of the isopropyl moiety in the N-aryl group.


 Please wait while we load your content...
Please wait while we load your content...