Highly cost-effective platinum-free anion exchange membrane electrolysis for large scale energy storage and hydrogen production
Abstract
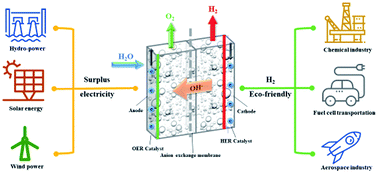
Anion exchange membrane (AEM) electrolysis eradicates platinum group metal electrocatalysts and diaphragms and is used in conventional proton exchange membrane (PEM) electrolysis and alkaline electrolysis. It can produce pressurised hydrogen by using low cost non-noble metal catalysts. However, the performances are still lower than that of the conventional PEM electrolysis technology. In this study, we addressed the performance issue by using a novel combination of Ni–Fe–Ox for oxygen evolution reaction (OER) and Ni–Fe–Co hydrogen evolution reaction (HER) electrodes with a PBI anion exchange membrane. The Ni–Fe–Ox and Ni–Fe–Co electrodes exhibit exceptionally high catalytic activity, requiring over potentials that are as low as 236 and 84 mV dec−1, respectively, for OER and HER to occur. These electrocatalysts exhibits excellent durability which can be used as oxygen evolution and hydrogen evolution catalysts for long term electrolysis. The high rate capability of 1000 mA cm−2 at 1.9 V and 60 °C demonstrates the potential of the combined membrane electrode assembly. The best performance, which is comparable to those of commercial PEM electrolysis systems, is thus an affordable alternative to this technology. In addition to that, the AEM electrolysis is promising on a multi-scale level for long-term hydrogen production.


 Please wait while we load your content...
Please wait while we load your content...