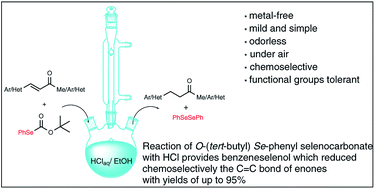
Chemoselective and metal-free reduction of α,β-unsaturated ketones by in situ produced benzeneselenol from O-(tert-butyl) Se-phenyl selenocarbonate†
Abstract
The carbon–carbon double bond of arylidene acetones and chalcones can be selectively reduced with benzeneselenol generated in situ by reacting O-(tert-butyl) Se-phenyl selenocarbonate with hydrochloric acid in ethanol. This mild, metal-free and experimentally simple reduction procedure displays considerable functional-group compatibility, products are obtained in good to excellent yields, and the use of toxic Se/CO mixture and NaSeH, or the smelly and air-sensitive benzeneselenol, is avoided.


 Please wait while we load your content...
Please wait while we load your content...