Carotane sesquiterpenes from Ferula vesceritensis: in silico analysis as SARS-CoV-2 binding inhibitors†
Abstract
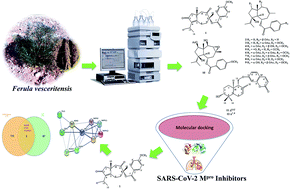
Two sesquiterpenes, 8α-anisate-dauc-4-ene-3,9-dione (webiol anisate) (1) and 10α-acetoxy-6α-benzoate-jaeschkeanadiol (2) as well as, ten known analogues (3–10), and two sesquiterpene coumarins (11–12) were isolated from an organic root extract of Ferula vesceritensis (Fam. Apiaceae). Chemical structures were elucidated based on IR, 1D- and 2D-NMR and HRMS, spectroscopic analyses. With molecular overlap observed between two protease inhibitors that are being examined as anti-COVID-19 drugs, and sesquiterpenes isolated here, metabolite molecular docking calculations were made using the main protease (Mpro), which is required for viral multiplication as well as RNA-dependent RNA polymerase (RdRp). In silico binding-inhibition analysis predicted that select F. vesceritensis sesquiterpenes can bind to these enzymes required for viral replication. Structures of the isolated constituents were also consistent with the chemo-systematic grouping of F. vesceritensis secondary metabolites with other Ferula species.
- This article is part of the themed collection: Coronavirus articles - free to access collection


 Please wait while we load your content...
Please wait while we load your content...