Comparative study of gold and silver interactions with amino acids and nucleobases†
Abstract
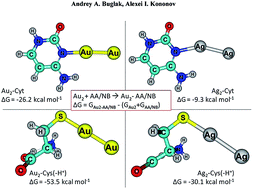
Metal nanoclusters (NCs) have gained much attention in the last decade. In solution, metal nanoclusters can be stabilized by proteins, and, thus, exhibit many advantages in biocatalysis, biosensing, and bioimaging. In spite of much progress in the synthesis of polypeptide-stabilized gold (Au) clusters, their structure, as well as amino acid-cluster and amino acid–Au+ interactions, remain poorly understood. It is not entirely clear which amino acid (AA) residues and sites in the protein are preferred for binding. The understanding of NC-protein interactions and how they evolve in the polypeptide templates is the key to designing Au NCs. In this work, binding of gold ion Au+ and diatomic neutral gold nanocluster Au2 with a full set of α-proteinogenic amino acids is studied using Density Functional Theory (DFT) and the ab initio RI-MP2 method in order to find the preferred sites of gold interaction in proteins. We demonstrated that the interaction of gold cations and clusters with protonated and deprotonated amino acid residues do not differ greatly. The binding affinity of AAs to the Au2 cluster increases in the following order: Cys(−H+) > Asp(−H+) > Tyr(−H+) > Glu(−H+) > Arg > Gln, His, Met ≫ Asn, Pro, Trp > Lys, Tyr, Phe > His(+H+) > Asp > Lys(+H+) > Glu, Leu > Arg(+H+) > Ile, Val, Ala > Thr, Ser > Gly, Cys, which agrees with the available experimental data that gold cluster synthesis occurs in a wide range of pH – amino acid residues with different protonation states are involved in this process. The significant difference in the binding energy of metal atoms with nucleobases and amino acids apparently means that unlike on DNA templates, neutral metal atoms are strongly bound to amino acid residues and can't freely diffuse in a polypeptide globula. This fact allows one to conclude that formation of metal NCs in proteins occurs through the nucleation of reduced Au atoms bound to the neighboring amino acid residues, and the flexibility of the amino acid residue side-chains and protein chain as a whole plays a significant role in this process.


 Please wait while we load your content...
Please wait while we load your content...