HKUST-1 derived Cu@CuOx/carbon catalyst for base-free aerobic oxidative coupling of benzophenone imine: high catalytic efficiency and excellent regeneration performance†
Abstract
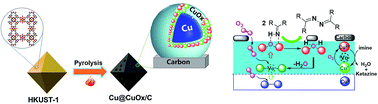
The oxidative coupling of imines to ketazine with molecular oxygen is a green process towards the synthesis of hydrazine or hydrazine hydrate, which could efficiently address the economic and environmental issues of the traditional Raschig or peroxide-ketazine process. Herein, we developed an efficient heterogeneous base-free benzophenone imine oxidative coupling route with O2 catalyzed by Cu/CuOx/carbon materials derived from MOFs under mild conditions. Under optimized conditions, the conversion of BI is up to 98.2% and the selectivity of ketamine is 94.9%. This catalyst has excellent structure stability, recycling, and regeneration performance, owing to the carbonization of organic ligands of MOF at high temperature. More importantly, it is confirmed that the metallic Cu core is essential to improve the catalytic performance of the CuO shell in the BI oxidative coupling reaction, due to the promotion of electron transfer in the CuO surface, making dissolved O2 molecules more easily insert oxygen vacancies. This strategy might open an avenue to the sustainable catalytic synthesis of hydrazine or hydrazine hydrate.


 Please wait while we load your content...
Please wait while we load your content...