Electrochemical oxidation of resorcinol: mechanistic insights from experimental and computational studies†
Abstract
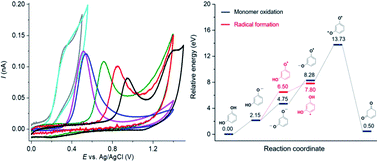
This work investigates the mechanisms of resorcinol oxidation by density functional theory (DFT) calculation and cyclic voltammetry measurements. Complementary data from experimental and computational studies provide new insights into the reaction mechanisms. At both macro- and micro-electrodes, cyclic voltammetry of resorcinol is chemically and electrochemically irreversible over the whole pH range (1–14). Resorcinol molecules undergo a 1H+ 1e− oxidation at pH < pKa1 and a 1e− oxidation at pH > pKa2 to form radicals. The radicals then readily react to form dimers/polymers deposited on the electrode surface. All of the experimental findings are consistent with the proposed mechanisms, including the apparent transfer coefficient (β) of 0.6 ± 0.1, the slope of the peak potential (Ep) against pH of −54 mV pH−1, the peak-shaped responses at micro-electrodes, and the fouling of the electrodes upon the oxidation of resorcinol. DFT calculation of the reaction energy of elementary steps and the eigenvalues of the highest occupied molecular orbital (HOMO) of the radical intermediates confirms that the (1H+) 1e− oxidation is the energetically favorable pathway. In addition to mechanistic insights, an electrochemical sensor is developed for resorcinol detection at microelectrodes in low ionic strength samples with the sensitivity of 123 ± 4 nA μM−1 and the limit of detection (3 sB m−1) of 0.03 μM.


 Please wait while we load your content...
Please wait while we load your content...