Recent advances and prospects in the palladium-catalyzed cyanation of aryl halides
Abstract
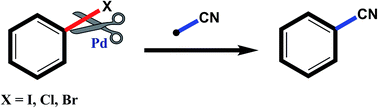
Aryl nitriles are compounds with wide significance. They have made their own space in various sectors including pharmaceuticals, industries, natural product chemistry, and so on. Furthermore, they are also key intermediates in various transformations in organic chemistry. Transition metal-catalyzed cyanation reactions have proved to be a better replacement for the existing traditional synthetic strategies for aryl nitriles. Palladium is one of the most studied transition metals other than copper and nickel owing to its wide functional group compatibility and catalytic efficacy. There have been drastic developments in the field of palladium-catalyzed cyanation since its discovery in the 1973. This review summarizes the recent developments in the palladium-catalyzed cyanation of aryl halides and covers literature from 2012–2020.
- This article is part of the themed collection: 2020 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...