Functionalized pyridine in pyclen-based iron(iii) complexes: evaluation of fundamental properties†
Abstract
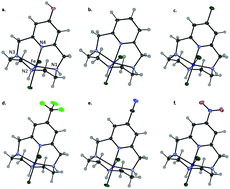
The use of tetra-aza pyridinophanes is of increasing interest in the fields of bioinorganic modeling, catalysis, and imaging. However, a full study of how modifications to the pyridyl moiety affect the characteristics of the daughter metal complexes, has not been explored. In this study, six tetra-aza macrocyclic ligands were metalated with Fe(III) and were characterized for the first time. The pyridyl functional groups studied include: 4-hydroxyl (L1), 4-H (L2), 4-chloro (L3), 4-trifluoromethyl (L4), 4-nitrile (L5), and 4-nitro (L6) modified pyridyl on a pyclen base structure. The resulting iron complexes were characterized by X-ray diffraction analysis, cyclic voltammetry, and metal-binding affinities (log β) were determined. Analysis of these results indicate that such functionalizations introduce a handle by which electrochemical properties and thermodynamic stability of daughter complexes with transition metal ions can be tuned, which in turn, could potentially impact the reactivity of these complexes in future studies.


 Please wait while we load your content...
Please wait while we load your content...