Investigation of the cis–trans structures and isomerization of oligoprolines by using Raman spectroscopy and density functional theory calculations: solute–solvent interactions and effects of terminal positively charged amino acid residues†
Abstract
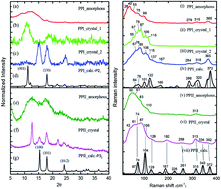
Using low-wavenumber Raman spectroscopy in combination with theoretical calculations via solid-state density functional theory (DFT)-D3, we studied the vibrational structures and interaction with solvent of poly-L-proline and the oligoproline P12 series. The P12 series includes P12, the positively charged amino acid residue (arginine and lysine) N-terminus proline oligomers RP11 and KP11, and the C-terminus P11R and P11K. We assigned the spring-type phonon mode to 74–76 cm−1 bands for the PPI and PPII conformers and the carbonyl group ring-opening mode 122 cm−1 in the PPI conformer of poly-L-proline. Amide I and II were assigned based on the results of mode analysis for O, N, and C atom displacements. The broad band feature of the H-bond transverse mode in the Raman spectra indicates that the positively charged proline oligomers PPII form H-bonds with water in the solid phase, whereas P12 is relatively more hydrophobic. In propanol, the PPI conformer of the P12 series forms less H-bond network with the solvent. The PPII conformer exhibits a distinct Raman band at 310 cm−1, whereas the PPI has bands at 365, 660, and 960 cm−1 with reasonable intensity that can be used to quantitatively determine these two conformational forms. The 365 cm−1 mode comprising the motion of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group turning to the helix axis was used to monitor the isomerization reaction PPI ↔ PPII. In pure propanol, RP11 and KP11 were found to have mostly PPI present, but P11R and P11K preferred PPII. After adding 20% water, the PPI in P11R and P11K was completely converted to PPII, whereas a small fraction of PPI remained in RP11 and KP11. The substituted positively charged amino acid affected the balance of the PPI/PPII population ratio.
O group turning to the helix axis was used to monitor the isomerization reaction PPI ↔ PPII. In pure propanol, RP11 and KP11 were found to have mostly PPI present, but P11R and P11K preferred PPII. After adding 20% water, the PPI in P11R and P11K was completely converted to PPII, whereas a small fraction of PPI remained in RP11 and KP11. The substituted positively charged amino acid affected the balance of the PPI/PPII population ratio.


 Please wait while we load your content...
Please wait while we load your content...