Purification and characterization of a thaumatin-like protein-1 with polyphenol oxidase activity found in Prunus mume†
Abstract
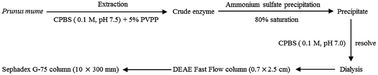
Thaumatin-like protein-1 (TLP-1), a protein displaying high polyphenol oxidase (PPO) action and a member of the pathogenesis-related (PR) protein family, has a considerable influence on the enzymatic browning of Prunus mume (Chinese plum). In this assay, TLP-1 was identified and extracted from Prunus mume to investigate the protein's properties and better understand its contribution to the fruit's browning during storage or processing. The extracted TLP-1 was purified to apparent homogeneity using a procedure involving citrate phosphate buffer solution (CPBS) extraction, (NH4)2SO4 precipitation, dialysis in a cellulose bag, and ion exchange chromatography using a DEAE Sepharose Fast Flow column, while a Sephadex G-75 column was employed to facilitate gel filtration chromatography. Moreover, the enzyme was characterized in terms of its optimal pH and stability, isoelectric point (pI), molecular weight, optimal temperature and stability, enzyme kinetic parameters and substrate specificity, as well as inhibitor stability. This study indicated that the pI and molecular weight of TLP-1 was approximately 4.4 and 28 kDa, respectively, while 30 °C and 7.5 represented the respective optimal temperature and pH level for PPO catalysis. TLP-1 showed high affinity to catechol and pyrogallol, with Km values of 24.40 mM and 26.23 mM, respectively. Sodium bisulfite significantly inhibited TLP-1 activity. These findings on the properties of TLP-1 can contribute significantly to the search for ways to minimize the losses caused by fruit browning during the storage and processing of Prunus mume.


 Please wait while we load your content...
Please wait while we load your content...