Helically shaped cation receptor: design, synthesis, characterisation and first application to ion transport†
Abstract
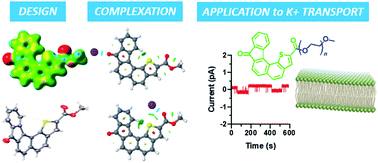
The methyl ester of 8-oxo-8H-indeno[2′,1′:7,8]naphtho[1,2-b]thiophene-2-carboxylic acid (1) and its corresponding PEGylated ester were synthesised and fully characterised. X-ray diffraction studies on (1) confirmed the helical structure of the receptor and that it is self-assembled into layers by π–π interactions. An in-depth study by DFT calculations and MS experiments (ESI-MS, MS/MS, IMRPD and ESI-IMS-MS) was carried out between (1) and the physiological cation K+. The formation of supramolecular complexes between (1) and K+ with different stoichiometries was demonstrated and the cation K+ preferentially interacts with the oxygen atoms of the carbonyl bond of the ketone and ester groups and the sulphur atom of the heterocycle. The ability of the two synthesized aromatic architectures to transport ions across a model lipid membrane has been studied by electrophysiology experiments. The formation of pores was observed, even at nanomolar concentrations. Since the PEGylated molecule showed more regular pore definitions than the hydrophobic molecule, the introduction of a polar hydrophilic chain made it possible to control the orientation of the aromatic architectures within the membrane.


 Please wait while we load your content...
Please wait while we load your content...