Density functional theory-based investigation of HCN and NH3 formation mechanisms during phenylalanine pyrolysis†
Abstract
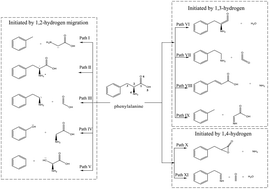
As sludge pyrolysis produces large amounts of toxic NH3 and HCN, many works have studied nitrogen transfer during this process, commonly employing amino acids as models of sludge protein. Herein, density functional theory is used to probe the production of HCN and NH3 during the pyrolysis of phenylalanine as a model, revealing the existence of two formation paths for each gas. In the first (lower-energy-barrier) NH3 formation path, the hydrogen bonding-assisted transfer of carboxyl group hydrogen to the amino group is followed by direct NH3 generation via decarboxylation, and the second (higher-energy-barrier) path features decarboxylation followed by the transfer of carboxyl group hydrogen to the adjacent carbon atom to form phenethylamine, the deamination of which affords NH3 and styrene. For HCN, the first (lower-energy-barrier) path features C2–C3 bond cleavage to afford dehydroglycine, which further decomposes to produce HCN, while in the second path, the decomposition of phenylalanine into phenethylamine, CO, and H2O is followed by internal hydrogen transfer in phenethylamine to generate HCN. The overall energy barrier of the two HCN formation paths exceeds that of NH3 formation paths, i.e., phenylalanine is more prone to afford NH3 than HCN upon pyrolysis.


 Please wait while we load your content...
Please wait while we load your content...