Transistor properties of salen-type metal complexes†
Abstract
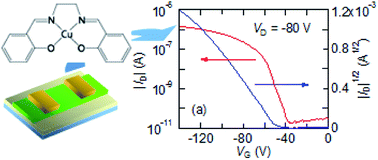
Schiff base complexes derived from salicylaldehyde and ethylene-, propylene-, and trans-1,2-cyclohexane-diamines exhibit p-channel transistor properties. The Cu complexes are open-shell compounds, but the oxidation and the hole transport occur at the highest occupied molecular orbital, where the singly occupied molecular orbital (SOMO) does not participate in conduction. Although Ni complexes tend to show larger mobilities than Cu complexes owing to the molecular planarity, the presence of SOMO is not harmful to the transistor properties.


 Please wait while we load your content...
Please wait while we load your content...