A guanosine-based 2-formylphenylborate ester hydrogel with high selectivity to K+ ions†
Abstract
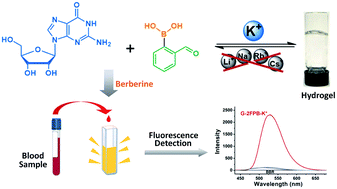
Guanosine-based supramolecular hydrogels are particularly of interest for biomaterial and biomedical purposes, as they are generally biocompatible and stimuli-responsive. We found a strong and long-life transparent hydrogel made by mixing guanosine (G) with 1 equiv. of 2-formylbenzeneboronic acid (2FPB) and KOH. Alkali cations can assist the stacking of individual G-quartet to give extended nanowires, but only K+ ion induces the formation of a stable and self-supporting network hydrogel for a couple of months. Data from variable temperature NMR indicated that guanosine 2-formylbenzeneborate ester and G are the key components of the self-assembly. Further, G-2FPB-K+ hydrogel solution can induce berberine (BBR) fluorescence, showing high selectivity to K+ ion and anti-ion interference capability. A good linear relationship between fluorescent intensity and K+ concentration allowed us to directly detect K+ levels in human blood serum.


 Please wait while we load your content...
Please wait while we load your content...