Facile deprotection of F-BODIPYs using methylboronic acid†
Abstract
4,4-Difluoro-4-bora-3a,4a-diaza-s-indacenes (F-BODIPYs) are deprotected through removal of the –BF2 moiety upon treatment with methylboronic acid. The tolerance of various substitution patterns about the dipyrrinato core is demonstrated via the deprotection of thirteen F-BODIPYs and an F-aza-BODIPY. Work-up with aq. HBr affords the desired dipyrin HBr salt in quantitative yield without need for purification.
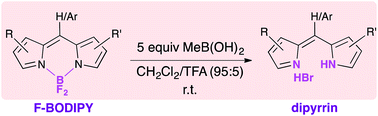


 Please wait while we load your content...
Please wait while we load your content...