Synthetically important ring opening reactions by alkoxybenzenes and alkoxynaphthalenes†
Abstract
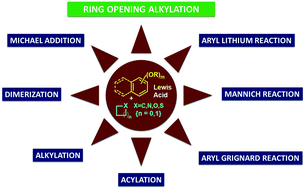
Alkoxybenzenes and alkoxynaphthalenes, as nucleophiles, have drawn great attention from organic chemists over the decades. Due to their high ring strain, those particular classes of molecules are often used in synthesis by utilizing their properties to undergo facile Friedel–Crafts alkylations. Different isomeric and low or densely substituted alkoxybenzenes are used for synthesis according to the structure of the target molecule. Isomeric methoxybenzenes, are the most commonly used molecule in this regard. This review aims to comprehensively cover the instances of different alkoxy-benzenes/naphthalenes used as nucleophiles for ring opening.
- This article is part of the themed collection: 2020 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...