Synthesis and biochemical evaluation of cephalosporin analogues equipped with chemical tethers†
Abstract
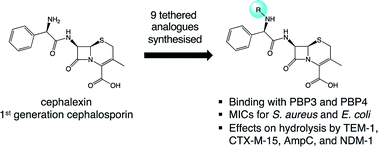
Molecular probes typically require structural modifications to allow for the immobilisation or bioconjugation with a desired substrate but the effects of these changes are often not evaluated. Here, we set out to determine the effects of attaching functional handles to a first-generation cephalosporin. A series of cephalexin derivatives was prepared, equipped with chemical tethers suitable for the site-selective conjugation of antibiotics to functionalised surfaces. The tethers were positioned remotely from the β-lactam ring to ensure minimal effect to the antibiotic's pharmacophore. Herein, the activity of the modified antibiotics was evaluated for binding to the therapeutic target, the penicillin binding proteins, and shown to maintain binding interactions. In addition, the deactivation of the modified drugs by four β-lactamases (TEM-1, CTX-M-15, AmpC, NDM-1) was investigated and the effect of the tethers on the catalytic efficiencies determined. CTX-M-15 was found to favour hydrolysis of the parent antibiotic without a tether, whereas AmpC and NDM-1 were found to favour the modified analogues. Furthermore, the antimicrobial activity of the derivatives was evaluated to investigate the effect of the structural modifications on the antimicrobial activity of the parent drug, cephalexin.


 Please wait while we load your content...
Please wait while we load your content...