Copper(ii)-catalyzed synthesis of multisubstituted indoles through sequential Chan–Lam and cross-dehydrogenative coupling reactions†
Abstract
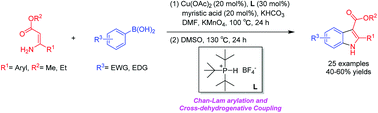
Starting from arylboronic acids and ester (Z)-3-aminoacrylates, one-pot syntheses of diverse indole-3-carboxylic esters have been described through copper(II)-catalyzed sequential Chan–Lam N-arylation and cross-dehydrogenative coupling (CDC) reactions. The initial Chan–Lam arylation can proceed in DMF at 100 °C for 24 h to give ester (Z)-3-(arylamino)acrylate intermediates in the presence of Cu(OAc)2/tri-tert-butylphosphine tetrafluoroborate, a catalytic amount of myristic acid as the additive, KMnO4 and KHCO3. Sequentially, these in situ arylated intermediates can undergo an intramolecular oxidative cross-dehydrogenative coupling process in mixed solvents (DMF/DMSO = 2 : 1) at 130 °C to give C3-functionalized multi-substituted indole derivatives.


 Please wait while we load your content...
Please wait while we load your content...