One-pot method for the synthesis of 1-aryl-2-aminoalkanol derivatives from the corresponding amides or nitriles†
Abstract
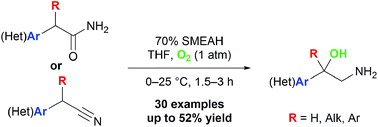
We have identified a novel one-pot method for the synthesis of β-amino alcohols, which is based on C–H bond hydroxylation at the benzylic α-carbon atom with a subsequent nitrile or amide functional group reduction. This cascade process uses molecular oxygen as an oxidant and sodium bis(2-methoxyethoxy)aluminum hydride as a reductant. The substrate scope was examined on 30 entries and, although the respective products were provided in moderate yields only, the above simple protocol may serve as a direct and powerful entry to the sterically congested 1,2-amino alcohols that are difficult to prepare by other routes. The plausible mechanistic rationale for the observed results is given and the reaction was applied to a synthesis of a potentially bioactive target.
- This article is part of the themed collection: 2020 RSC Advances HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...