Effect of substituents and promoters on the Diels–Alder cycloaddition reaction in the biorenewable synthesis of trimellitic acid†
Abstract
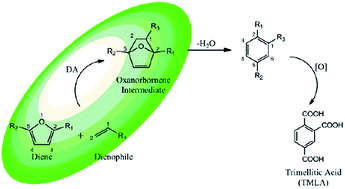
An efficient route to produce oxanorbornene, a precursor for the production of bio-based trimellitic acid (TMLA) via the Diels–Alder (DA) reaction of biomass-derived dienes and dienophiles has been proposed by utilizing density functional theory (DFT) simulations. It has been suggested that DA reaction of dienes such as 5-hydroxymethyl furfural (HMF), 2,5-dimethylfuran (DMF), furan dicarboxylic acid (FDCA) and biomass-derived dienophiles (ethylene derivatives e.g., acrolein, acrylic acid, etc.) leads to the formation of an intermediate product oxanorbornene, a precursor for the production of TMLA. The activation barriers for the DA reaction were correlated to the type of substituent present on the dienes and dienophiles. Among the dienophiles, acrolein was found to be the best candidate showing a low activation energy (<40 kJ mol−1) for the cycloaddition reaction with dienes DMF, HMF and hydroxy methyl furoic acid (HMFA). The FMO gap and (IPdiene + EAdienophile)/2 were both suggested to be suitable descriptors for the DA reaction of electron-rich diene and electron-deficient dienophile. Further solvents did not have a significant effect on the activation barrier for DA reaction. In contrast, the presence of a Lewis acid was seen to lower the activation barrier due to the reduction in the FMO gap.


 Please wait while we load your content...
Please wait while we load your content...