Phosphorus pentoxide as a cost-effective, metal-free catalyst for ring opening polymerization of ε-caprolactone†
Abstract
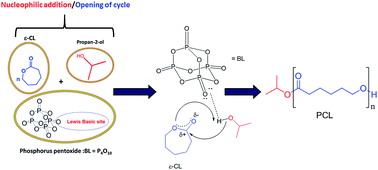
The ring-opening polymerization (ROP) of ε-caprolactone (ε-CL) using phosphorus pentoxide (P2O5) as a metal-free catalyst and isopropanol (iPrOH) as initiator resulted in the preparation of poly(ε-caprolactone) with narrow weight distribution. NMR spectroscopy analyses of the prepared PCL indicated the presence of the initiator residue at the end of the polymer chain, implying the occurrence of the ε-CL-catalysis ROP through a monomer activation mechanism. Kinetic experiments confirmed the controlled/living nature of ε-CL ring-opening catalyzed by phosphorus pentoxide. The commercial availability of phosphorus pentoxide and its easy-handling provide additional opportunities for polymer synthesis and nanocomposite manufacturing.


 Please wait while we load your content...
Please wait while we load your content...