K2S2O8-mediated radical cyclisation of 2-alkynylthioanisoles or -selenoanisoles: a green and regioselective route to 3-nitrobenzothiophenes and benzoselenophenes†
Abstract
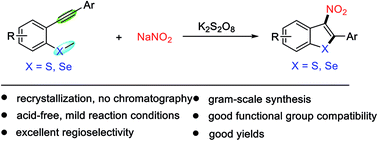
An acid, transition-metal, and chromatography-free radical nitration/cyclisation of 2-alkynylthioanisoles or -selenoanisoles has been developed. This is the first example of the use of highly unstable 2-nitrovinyl radicals for C–S bond formation. This facile route efficiently produces 3-nitrobenzothiophenes and benzoselenophenes, which are difficult to access via classical methods. Density functional theory (DFT) calculations were carried out to probe the reaction mechanism. The resulting products were tested for their in vitro anti-tuberculosis activity, and compounds 2d and 2l showed significant activities against sensitive and drug-resistant strains.


 Please wait while we load your content...
Please wait while we load your content...