A highly green approach towards aromatic nitro group substitutions: catalyst free reactions of nitroimidazoles with carbon nucleophiles in water†
Abstract
We have successfully developed a flexible green aqueous approach for the formation of a carbon–carbon bond by the reaction of highly-enolizable carbanions (mostly derived from 1,3-dicarbonyl compounds) with an aromatic carbon bearing a nitro group. The key step involves a nucleophilic displacement reaction. All newly synthesized compounds were unambiguously characterized via various spectroscopic techniques including NMR, mass spectrometry and single-crystal X-ray diffraction as applicable. We believe that our study will be useful in providing new insights into catalyst-free water-mediated nucleophilic substitution reactions.
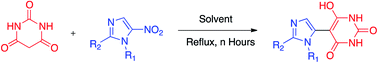


 Please wait while we load your content...
Please wait while we load your content...