Thermokinetic behaviour and functional group variation during spontaneous combustion of raw coal and its preoxidised form
Abstract
Coal spontaneous combustion (CSC) is a major problem in coal mining. In the vicinity of underground goaf, secondary or repeated oxidation processes of the residual coal inevitably occur, increasing the risk of coal fires. In this study, the thermal reaction behaviour of two types of raw coal samples and three preoxidised coal samples with different oxidation temperatures (80, 130, and 180 °C) were investigated. The physical and chemical properties of the samples were measured using thermogravimetric analyser-Fourier transform infrared spectroscopy (TGA-FTIR) with heating rates of 1.0, 2.0, 5.0, and 10.0 °C min−1. According to the characteristic temperatures in the heating processes, the entire CSC procedure can be divided into three stages: oxidation, combustion, and burnout. The results indicated that the aliphatic side chain lengths of preoxidised coal were shorter, and the number of branched aliphatic side chains was lower than that of raw coal. Furthermore, the model for the mechanism of preoxidised coal differed from that of raw coal. Average values of the apparent activation energy 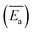 of the preoxidised coal samples were lower than those of the raw coal samples. Therefore, compared with raw coal, preoxidised coal requires less energy to react and more readily undergoes spontaneous combustion.
of the preoxidised coal samples were lower than those of the raw coal samples. Therefore, compared with raw coal, preoxidised coal requires less energy to react and more readily undergoes spontaneous combustion.



 Please wait while we load your content...
Please wait while we load your content...