Green and facile synthesis of water-soluble carbon dots from ethanolic shallot extract for chromium ion sensing in milk, fruit juices, and wastewater samples†
Abstract
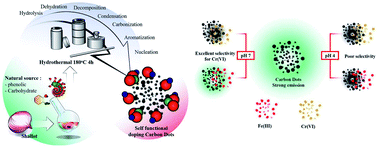
Self-functionalized carbon dots (CDs) were prepared from ethanolic shallot extract to obtain a total phenolic precursor. The total phenolic extract was then heated at 180 °C for four hours in an autoclave. Only 1 mg L−1 of CDs had high fluorescence emission at 430 nm after excitation at 340 nm and manifested a high selectivity for Cr(VI) ions. The inter- and intra-day emission stability, pH, ionic strength, solvent effect, Stern–Volmer constant, incubation time, speciation of Cr(III) and Cr(VI) ions, and ion selectivity of the as-prepared CDs were investigated in detail. The proposed method was validated in 20–100 μM linearity with y = 2.2346x as the set-zero intercept linear equation, 0.9981 as the correlation coefficient, 3.5 μM as the limit of detection (LOD), 11.7 μM as the limit of quantification (LOQ), and 2.78% and 5.29% as the intra-day and inter-day relative standard deviations (RSD), respectively. The recovery of drinking water, milk, soymilk, fruit juices (apple and coconut), tap water, and chromium-coated industrial waste water by the investigated Cr sensor was found to be 78.58–119.69%. Therefore, the proposed Cr(VI) sensor had superior advantages of sensitivity, selectivity, rapidity, and reproducibility.


 Please wait while we load your content...
Please wait while we load your content...