Aqueous Cu(ii) ion adsorption by amino-functionalized mesoporous silica KIT-6
Abstract
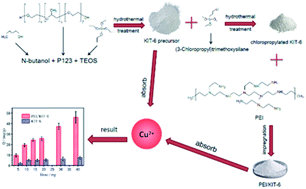
To find an alternative adsorbent with high adsorption performance, KIT-6 was prepared by hydrothermal crystallization synthesis using tetraethyl orthosilicate as a silicon source and triblock copolymer P123 as a template. Then the silane coupling agent (3-chloropropyl)trimethoxysilane was first grafted onto KIT-6 mesoporous material and then the polyethyleneimine (PEI) was further grafted through the substitution reaction between amino groups and chlorine atoms. The functionalized KIT-6 was denoted as PEI/KIT-6. The samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), N2 adsorption–desorption, elemental analysis (EA), Fourier transform infrared spectroscopy (FT-IR) and thermal gravimetric analysis (TGA). The Cu2+ adsorption performance was determined by inductively coupled plasma (ICP). The results showed that the average loading of the amino groups was 3.74 mmol g−1, and the modified KIT-6 still has a stable mesoporous structure without pore blockage. With the dosage of 1 g L−1 PEI/KIT-6 and at room temperature, the optimum pH value for adsorption of 100 mg L−1 Cu2+ was 6.0. The adsorption capacity of PEI/KIT-6 for Cu2+ increased with the increase of reaction temperature, and the maximum adsorption capacity of Cu2+ was 36.43 mg g−1. The adsorption capacity tends to reach equilibrium after 120 min, and the optimum adsorption temperature was 35 °C. The pseudo-second-order kinetic model was found to be well suited for the adsorption process of Cu2+. Adsorption equilibrium data could also be described well by the classical Langmuir and Freundlich isotherm models. The adsorption tends to be the chemisorption of a monolayer.


 Please wait while we load your content...
Please wait while we load your content...