Deep insight into reasons for the mechanoluminescence phenomenon of triphenylamine-substituted imidazoles and their distinct mechano-fluorochromic behaviours†
Abstract
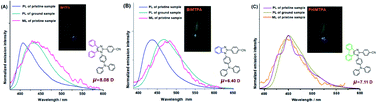
Three imidazoles with different numbers of fused aromatic rings have been prepared by the respective introduction of triphenylamine and 4-cyanophenyl at the N1 and C2 positions in the imidazole ring. Each imidazole effectively exhibits positive solvatochromism, and that of benzo[d]imidazole is the most significant. Although these imidazole crystals have centrosymmetric space groups, they are all ML-active. It was verified by DFT calculations based on X-ray crystallography that some molecular couples with strong intermolecular interactions possess large net dipole moments that should be dominantly responsible for the ML behaviours of these crystals. Moreover, the considerably high molecular dipole moments of the three imidazoles also make a great contribution to good ML effects. Based on this triphenylamine-substituted imidazole system, the relationships among space groups, molecular dipole moments, polar molecular couples and the ML phenomenon are made clear for the first time. Unlike the remarkable MFC activities on imidazole and benzo[d]midazole crystals, phenanthro[9,10-d]imidazole is MFC-inert, and this may well be attributed to strong intramolecular C–H⋯π interactions, which make the rotation of triphenylamine nearly impossible under force stimuli.


 Please wait while we load your content...
Please wait while we load your content...