RsmG forms stable complexes with premature small subunit rRNA during bacterial ribosome biogenesis†
Abstract
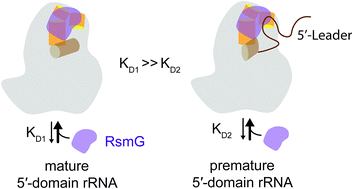
The ribosome is the ribonucleoprotein machine that carries out protein biosynthesis in all forms of life. Perfect synchronization between ribosomal RNA (rRNA) transcription, folding, post-transcriptional modification, maturation, and assembly of r-proteins is essential for the rapid formation of structurally and functionally accurate ribosomes. Many RNA nucleotide modification enzymes may function as assembly factors that oversee the accuracy of ribosome assembly. The protein RsmG is a methyltransferase enzyme that is responsible for N7 methylation in G527 of 16S rRNA. Here we illustrate the ability of RsmG to bind various premature small subunit ribosomal RNAs with contrasting affinities. Protein RsmG binds with approximately 15-times higher affinity to premature 16S rRNA with the full leader sequence compared to that of mature 16S rRNA. Various r-proteins which bind to the 5′-domain influence RsmG binding. The observed binding cooperativity between RsmG and r-proteins is sensitive to the maturation status of premature small subunit rRNA. However, neither the maturation of 16S rRNA nor the presence of various r-proteins significantly influence the methylation activity of RsmG. The capability of RsmG to bind to premature small subunit rRNA and alter its binding preference to various RNA–protein complexes based on the maturation of rRNA indicates its ability to influence ribosome assembly.


 Please wait while we load your content...
Please wait while we load your content...