Chlorotrifluoroethylidenes: an efficient and convenient approach to their synthesis†
Abstract
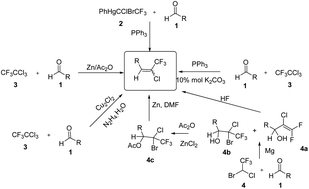
A convenient one step synthesis of chlorotrifluoroalkyl olefins starting from aldehydes was developed. The stable reagent 2-((1-chloro-2,2,2-trifluoroethyl)sulfonyl)benzothiazole was prepared from readily available benzothiazole-2-thiol and halothane. This method comprises using stable 2-((1-chloro-2,2,2-trifluoroethyl)sulfonyl)benzothiazole according to the Julia procedure and presents new opportunities for the synthesis of trifluoroalkylidene derivatives.


 Please wait while we load your content...
Please wait while we load your content...