Thermal decomposition and diffusion of methane in clathrate hydrates from quantum mechanics simulations
Abstract
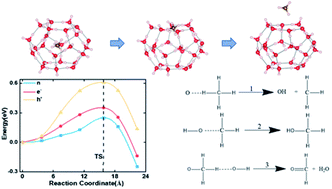
Clathrate hydrates are ice-like crystalline substances in which small gas molecules are trapped inside the polyhedral cavities of water molecules. They are of great importance in both scientific research and the petroleum industry because of their applications in modern energy and environmental technologies. To achieve an atomistic-level understanding of the diffusion and decomposition of trapped molecules in clathrate hydrate, we used methane hydrates (MHs) as the prototype system and examined the methane diffusion and decomposition mechanism by employing quantum mechanics (QM) and quantum mechanics molecular dynamics (QMD) simulations. Our QMD simulations illustrated that the initial decomposition reaction in MHs initiates from hydrogen transfer among water molecules and attacks by fragments of O and OH on CH4 molecules are responsible for the destruction of the methane molecules. Next, our QM simulations revealed that the methane molecule prefers to escape from the ice cage through the hexagonal face at low temperature. To suppress the methane diffusion, we demonstrated that the diffusion barrier is significantly enhanced by adding electron or hole carriers. This is because the extra electrons and holes enhance the electrostatic interaction between methane and water molecules, leading to an increased diffusion barrier. Thus, the clathrate hydrates could be stabilized by adding extra free electron or hole carriers.


 Please wait while we load your content...
Please wait while we load your content...