A chemically bonded supercapacitor using a highly stretchable and adhesive gel polymer electrolyte based on an ionic liquid and epoxy-triblock diamine network†
Abstract
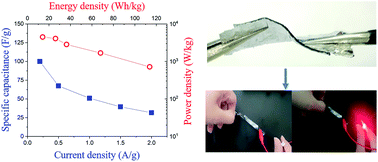
Despite significant advances in the development of flexible gel polymer electrolytes (GPEs), there are still problems to be addressed to apply them to flexible electric double layer capacitors (EDLCs), including interfacial interactions between the electrolyte and electrode under deformation. Previously reported EDLCs using GPEs have laminated structures with weak interfacial interactions between the electrode and electrolyte, leading to fragility upon elongation and low power density due to lower utilization of the surface area of the carbon material in the electrode. To overcome these problems, we present a new strategy for constructing an epoxy-based GPE that can provide strong adhesion between electrode and electrolyte. The GPE is synthesized by polymerization of epoxy and an ionic liquid. This GPE shows high flexibility up to 509% and excellent adhesive properties that enable strong chemical bonding between the electrode and electrolyte. Moreover, the GPE is stable at high voltage and high temperature with high ionic conductivity of ∼10−3 S cm−1. EDLCs based on the developed GPE exhibit good compatibility between the electrode and electrolyte and work properly when deformed. The EDLCs also show a high specific capacitance of 99 F g−1, energy density of 113 W h kg−1, and power density of 4.5 kW g−1. The excellent performance of the GPE gives it tremendous potential for use in next generation electronic devices such as wearable devices.


 Please wait while we load your content...
Please wait while we load your content...