Abiotic reduction of p-chloronitrobenzene by sulfate green rust: influence factors, products and mechanism†
Abstract
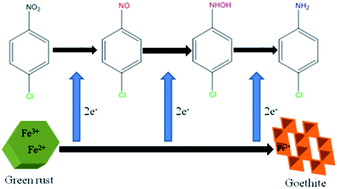
The reduction of p-chloronitrobenzene (p-CNB) by sulfate green rust (GRSO4) was systematically studied. The results revealed that GRSO4 has a good removal effect on p-CNB. The removal efficiencies of p-CNB by GRSO4 improved with the increase of the pH value. The removal efficiencies in the presence of ions were better than that of GRSO4 alone, while natural organic matter (NOM) could adsorb p-CNB, which competed with GRSO4. The reductions of p-CNB by GRSO4 under different conditions followed pseudo-first-order reaction kinetics except for the reactions in the presence of NOM. p-CNB was converted into p-chloroaniline (p-CAN), which produced p-nitrosochlorobenzene and p-chlorophenylhydroxylamine as the intermediate products. The results of the X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) showed GRSO4 was gradually transformed into goethite. Fe(II) in the GRSO4 structure was the main electron donor involved in the reaction.


 Please wait while we load your content...
Please wait while we load your content...