Synergistic effect of potassium iodide and sodium dodecyl sulfonate on the corrosion inhibition of carbon steel in HCl medium: a combined experimental and theoretical investigation
Abstract
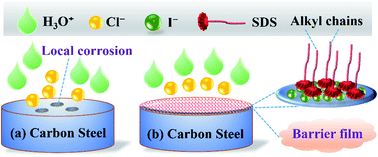
Carbon steel is an important industrial material, but it usually suffers from serious corrosion in the service environment. Using corrosion inhibitors is an effective approach to mitigate corrosion. The synergistic inhibition behavior of sodium dodecyl sulfonate (SDS) and potassium iodide (KI) on carbon steel corrosion in hydrochloric acid medium was investigated by electrochemical test, surface morphology analysis, and molecular simulation approaches. Results show that the corrosion inhibition performance is significantly enhanced after the two substances are compounded, and the inhibition efficiency can reach approximately 96% at small doses. The Tafel polarization curves suggest that the mixtures can be classified as anodic corrosion inhibitors. Impedance tests indicate that the inhibitor molecules are adsorbed on the steel surface, resulting in an increase of charge transfer resistance but a decrease of electric double layer capacitance. The adsorption process follows the Langmuir adsorption isotherm. Molecular simulation calculations further reveal the active sites of SDS and the stabilizing effect that I− plays in the inhibition process. The present research offers an economic, environmentally friendly and efficient measure of corrosion control, and provides theoretical guidance for the efficient use of carbon steels and the development of novel corrosion inhibitors.


 Please wait while we load your content...
Please wait while we load your content...