A concise and sequential synthesis of the nitroimidazooxazole based drug, Delamanid and related compounds†
Abstract
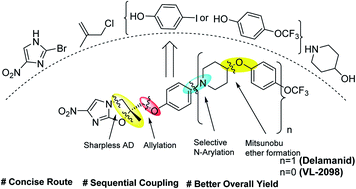
A concise, protection-group free and sequential route has been developed for the synthesis of the nitroimidazole based FDA-approved multi-drug resistant anti-tuberculosis drug, Delamanid and anti-leishmanial lead candidate VL-2098. The synthesis required chiral epoxides (11 and 17) as key intermediates. The chiral epoxide 11 was synthesised by sequential reaction cascades viz., allylation, selective N-arylation, Mitsunobu etherification, Sharpless asymmetric dihydroxylation and epoxidation, which do not require any special/dry reaction conditions. The steps involved towards the synthesis of epoxide also worked nicely in gram scales. After the synthesis of epoxide 11, the synthesis of Delamanid was achieved by reaction with 2-bromo-4-nitroimidazole 12 with an overall yield of 27%. Similarly, anti-leishmanial lead candidate VL-2098 was also synthesized in an overall yield of 36%.


 Please wait while we load your content...
Please wait while we load your content...