Atomic thermodynamics and microkinetics of the reduction mechanism of electrolyte additives to facilitate the formation of solid electrolyte interphases in lithium-ion batteries†
Abstract
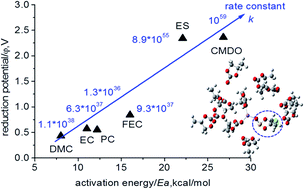
The formation of a solid electrolyte interphase (SEI) between the anode surface and the electrolyte of lithium-ion batteries (LIBs) has been considered to be the most important yet the least understood issue of LIBs. To further our understanding in this regard, the density functional theory (DFT) B3PW91/6-311++G(3df,3pd) together with the implicit solvent model and the transition state theory were used for the first time to comprehensively explore the electroreduction mechanism of a novel additive, 4-chloromethyl-1,3,2-dioxathiolane-2-oxide (CMDO), and a few other solvents and additives, such as ethylene carbonate (EC), propylene carbonate (PC), dimethyl carbonate (DMC), fluoroethylene carbonate (FEC), and even ethylene sulfite (ES), for comparison. The one-electron reduction potential of Li+-coordinated compounds Li+(X) for forming decomposition precursors [c-Li+(X˙−)] decreases in the following sequence: CMDO (1.9–2.2 V vs. Li+/Li) ∼ ES(1.9 V) > FEC (0.7 V) > EC (0.47 V) > PC (0.45 V) > DMC (0.38 V); this implies that CMDO is reduced prior to other solvents or additives in the mixture. Although the ring opening of [c-Li+(CMDO˙−)] is the least kinetically favorable, as reflected by the highest energy barrier (Ea), i.e., CMDO (18.8–22.9 kcal mol−1) ∼ ES (23.4) > FEC (16.2) > PC (12.5) > EC (11.2) > DMC (8.0), CMDO still shows the highest overall reaction rate constant (∼1053 s−1) for forming an open ring radical [o-Li+(CMDO˙)−]. In addition, the termination reaction of [o-Li+(CMDO˙)−] for forming LiCl is thermodynamically more favorable than that of Li2SO3 or organic disulfite (LiSO3)2-R, which supports the experimental observation that the halogen-containing LiF or LiCl additives are predominant over all other halogen-containing species in the SEI layer. Moreover, the hybrid model by including the second solvation shell of Li+ via a supercluster [(CMDO)Li+(PC)2](PC)9 and the implicit solvent model (SMD) can result in a reduction potential (∼1.7 V) that is in excellent agreement with the experimental reduction peak.


 Please wait while we load your content...
Please wait while we load your content...