Massive red shift of Ce3+ in Y3Al5O12 incorporating super-high content of Ce
Abstract
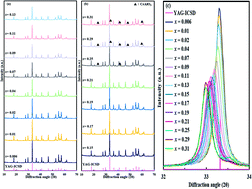
In light emitting diodes, Y3Al5O12:Ce (YAG:Ce) is used as a yellow phosphor in combination with blue LEDs but lacks a red component in emission. Therefore, considerable efforts have been directed toward shifting the emission of YAG:Ce to longer wavelengths. In this study, a Y3Al5O12 (YAG) crystal incorporating a high content of Ce, (Y1−xCex)3Al5O12 (0.006 ≦ x ≦ 0.21), was successfully prepared by a polymerized complex method in which low-temperature annealing (650–750 °C) was employed prior to sintering at 1080 °C. X-ray diffraction (XRD) and transmission electron microscopy (TEM) analysis indicated that the obtained sample was a single phase YAG crystal with x ≤ 0.21. Interestingly, orange-red emission was observed with x ≥ 0.07 with UV-blue light irradiation. With excitation at 450 nm, the emission peak increases from 538 nm (x = 0.006) to 606 nm (x = 0.21). This massive red shift in the high-x region was not observed without the 1st step of low-temperature annealing, which implied that low-temperature annealing was essential for incorporating a high concentration of Ce. The precursor formed by low-temperature annealing was amorphous at x = 0.04, whereas CeO2 nanocrystals were formed in the amorphous material with x ≥ 0.11, based on the XRD and TEM results. CeLIII X-ray absorption edge structure revealed that Ce existed as Ce4+ in the precursor and Ce3+ in the obtained crystal. It was speculated that CeO2 was formed at low temperature, releasing oxygen, with sintering at 1080 °C, leading to the incorporation of Y3+ in the Ce–O framework. The lattice constant increased significantly from 12.024 Å to 12.105 Å with increasing x, but the crystal field splitting did not increase and was constant from x = 0.06 to x = 0.21. Hence, the massive red shift in emission was not explained by the large crystal field splitting, but instead by the Stokes shift.


 Please wait while we load your content...
Please wait while we load your content...