One-pot copper-catalyzed three-component reaction: a modular approach to functionalized 2-quinolones†
Abstract
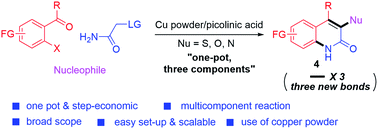
A copper-catalyzed three-component annulation for the synthesis of functionalized 2-quinolones was developed. Three reactions including an SN2, a Knoevenagel, and finally C–N bond formation are involved in the designed cascade reaction using 2-bromoacylarenes, 2-iodoacetamide, and nucleophiles as the three components. A new catalytic system was discovered during the study and this modular approach is highly efficient to access functionalized 2-quinolone derivatives, compatible with a broad range of functional groups, scalable, and step-economic. Further derivatization of the obtained product demonstrates the synthetic utility of this method.


 Please wait while we load your content...
Please wait while we load your content...