Electrophilic alkylation of arenes with 5-bromopyrimidine en route to 4-aryl-5-alkynylpyrimidines†
Abstract
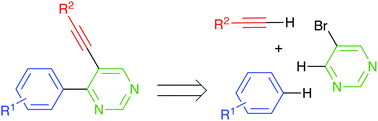
A new synthetic protocol for preparation of medicinally important 4-aryl-5-alkynylpyrimidines is described. The featured approach involves a sequence of chemo- and regioselective Brønsted acid-catalyzed electrophilic alkylation of arenes with 5-bromopyrimidine, followed by oxidative re-aromatization of the formed dihydropyrimidine ring. Finally, palladium-catalyzed Sonogashira cross-coupling reaction provided an end-game strategy.


 Please wait while we load your content...
Please wait while we load your content...