Microwave-assisted synthesis, biological evaluation and molecular docking studies of new coumarin-based 1,2,3-triazoles†
Abstract
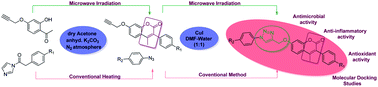
Coumarin-based 1,4-disubstituted 1,2,3-triazole derivatives were synthesized using a highly efficient, eco-friendly protocol via a copper(I)-catalyzed click reaction between various substituted arylazides and terminal alkynes. The synthetic route was easy to access and gave excellent yields under microwave irradiation conditions compared to the conventional heating route. The structures of all the compounds were characterized by IR, 1H NMR, 13C NMR spectroscopy and mass spectrometry. All the synthesized compounds were screened for their in vitro antimicrobial, antioxidant and anti-inflammatory activities; among all compounds, 8a, 8j, 8k and 8l exhibited better results with respect to standard drugs. Furthermore, molecular docking studies have been carried out with PDB IDs 2VCX (anti-inflammatory), 3VXI (antioxidant), 4GEE (antimicrobial) and 2XFH (antifungal) using the Glide module of the Schrödinger suite. The final compounds 8d, 8e, 8h, and 8k showed the highest hydrogen bond interactions with His-88 and Val-191 proteins and with water in all the proteins.
- This article is part of the themed collection: A Decade of Progress in Click Reactions Based on CuAAC


 Please wait while we load your content...
Please wait while we load your content...