A hydrazide organogelator for fluoride sensing with hyperchromicity and gel-to-sol transition†
Abstract
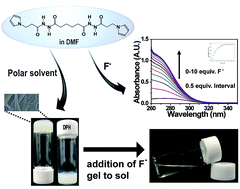
Sensing of fluoride in a solvent is highly required in healthcare and environmental rehabilitation. Among the various sensing methods, optical sensing has attracted significant research interest because it can conveniently recognize fluoride. Herein, a low molecular weight organogelator, N′1,N′6-bis(3-(1-pyrrolyl)propanoyl) hexanedihydrazide (DPH), containing a central butyl chain conjugated to two pyrrole rings through hydrazide groups, was used for optical sensing of fluoride in the forms of both solution and organogel. Association of fluoride with the –NH moiety of the hydrazide group endowed the DPH solution in dimethylformamide with a hyperchromicity under 350 nm. Exploiting the UV absorptivity, the DPH solution was examined as a chemosensor, displaying good selectivity toward fluoride among various anions and moderate sensitivity with a detection limit of 0.49 μM. The practical use of the DPH solution was demonstrated for fluoride sensing in toothpaste. Binding of fluoride also changed the molecular interactions of the DPH organogel, resulting in a phase transition from gel to sol. This gel-to-sol transition enabled the sensing of fluoride by the naked eye.


 Please wait while we load your content...
Please wait while we load your content...