An improved synthesis of telmisartan via the copper-catalyzed cyclization of o-haloarylamidines†
Abstract
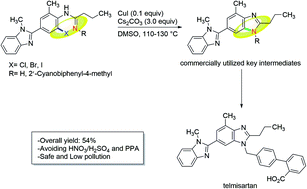
A concise synthetic route was designed for making telmisartan. The key bis-benzimidazole structure was constructed via the copper-catalyzed cyclization of o-haloarylamidines. By adopting this approach, telmisartan was obtained in a 7-step overall yield of 54% starting from commercially available 3-methyl-4-nitrobenzoic acid, and the use of HNO3/H2SO4 for nitration and polyphosphoric acid (PPA) for cyclization in the reported literatures were avoided.


 Please wait while we load your content...
Please wait while we load your content...